Expertise
5 tips for entering the Chinese medical technology market
From a statistical and economic point of view, China is the ideal country for investments in the field of medical technology and medical instruments, as we described previously in "The medical device market in China: opportunities and challenges" described earlier.

However, before you enter the promised land of double-digit growth, you should familiarize yourself thoroughly with the subject matter. Think about changing technical requirements on the one hand and cultural and, in particular, language barriers on the other. Today, we present some basic steps for a quick and smooth market entry.
1. research thoroughly (or ask experts)
First of all, make sure that there is sufficient demand for your product on the Chinese market. Consumer and hospital needs are changing rapidly in China. Therefore, follow the trends and analyze whether your product is competitive or fills a gap in the market. Keep in mind that public hospitals in China are encouraged to buy local products. Only in the event that no suitable alternatives are available from a Chinese manufacturer can they buy medical technology from the West. This means that your chances are best if you can offer innovative, high-quality products.
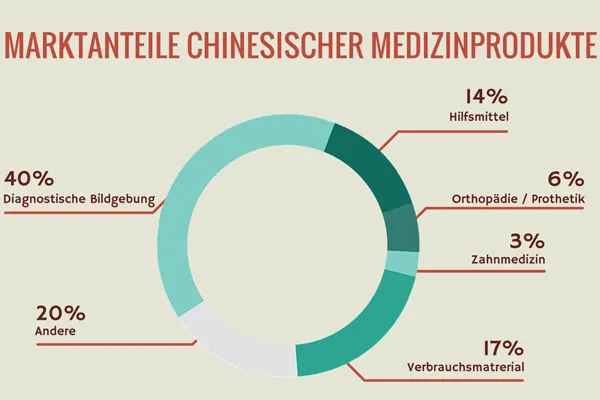
It is a good idea to carry out at least a simple basic survey regarding the outstanding functions of your device. What medical professionals in the West value is not necessarily at the top of the list for their colleagues in China. Before entering the market, it is crucial to conduct interviews with opinion leaders and potential users. Be sure to select reputable and professional scientific organizations to obtain representative results.
2 Who is who?
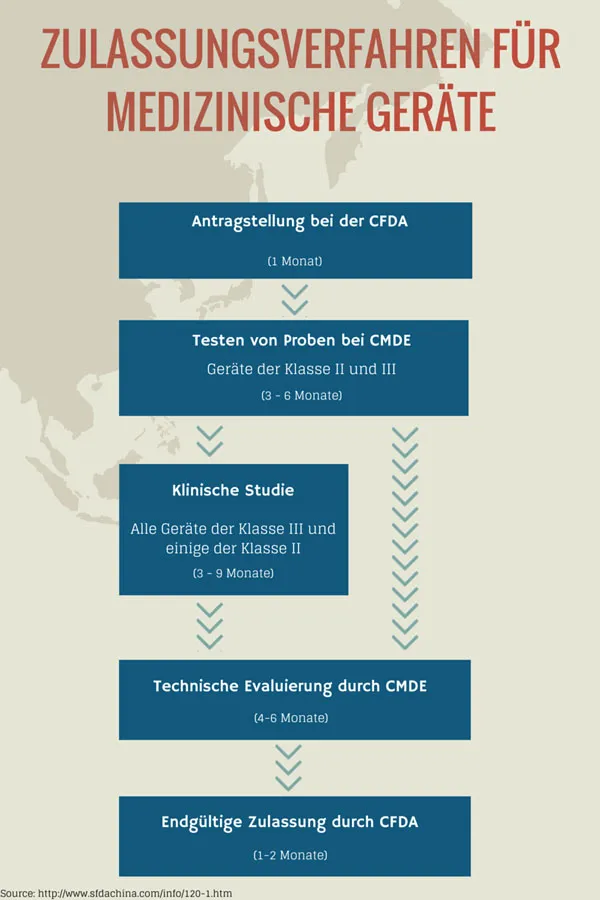
Identify the key players in your market. By this we mean not only your direct competitors, but also government agencies and institutions that are responsible for the registration and approval of your device. This is particularly important because the processes and approval procedures are more complicated and take longer than in Europe or North America.
- The Chinese Food and Drug Administration (CFDA) is the most important institution at ministerial level for entering the medical and health business in China. The CFDA is responsible for approval procedures for medical devices and instruments, the drafting of new regulations and rules, and safety and supervision in China.
- The Center for Medical Device Evaluation (CMDE) is responsible for conducting tests on medical devices during the approval process.
- The General Administration of Quality Supervision, Inspection, and Quarantine (AQSIQ) is responsible for the mandatory registration, approval and inspection of medical devices.
Visit the websites (especially the CFDA website) for new regulations and updates.
3. familiarize yourself with the approval procedure
According to CFDA regulations, medical devices refer to
"to instruments, equipment, tools, materials and other objects, including software, intended for use on the human body, either alone or in combination. These devices are used in the following applications:
- Prevention, diagnosis, treatment, monitoring or remission of diseases;
- Diagnosis, treatment, monitoring, remission or compensation for injury or physical disability;
- Research, exchange, adjustment of anatomical or physiological processes;
- Pregnancy control."
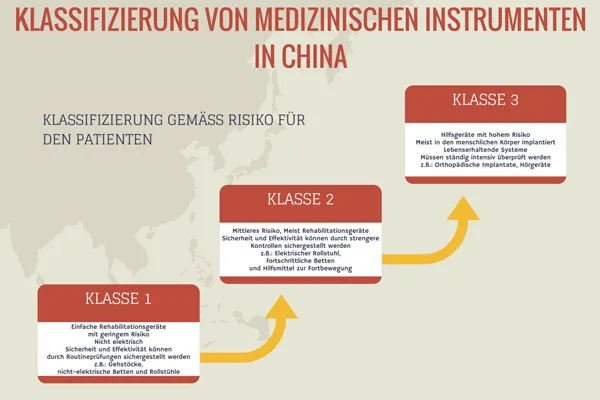
Before you contact the CFDA, you must determine the product class of your device. There are three product classes. Graded according to risk, this ranges from Class I (low risk, simple maintenance, non-electrical) to Class III (high potential risk, normally implanted in the human body or in the field of life support).
The approval process, as mentioned above, is arduous and time-consuming. It takes at least 14 months from application to approval. Clinical studies are necessary for highly developed devices. Approval in the country of origin is required.
4. plan design and development according to requirements from the outset
WILDDESIGN, familiar with the design of medical devices for more than two decades, knows only too well the tricky paths of product development in medical technology.
It is very important to carry out the design process in accordance with CFDA regulations from the very beginning, as the entire process is integrated. For CFDA approvals (as well as for FDA or CE), not only the design results are important, but also that the design process is carried out in accordance with the approval regulations. This means that from the very beginning, every design decision must be documented in the design history file and the usability considerations in the usability engineering file.
With a properly documented development process, a clear graphical user interface and good usability, you are one step closer to approval, avoiding rejection of your application and thus costly delays. Your chances of meeting your customers' expectations increase.
5. find trustworthy and professional sales partners
The CFDA requires three local authorities for marketing in China:
- Registration agent - the company that registers the medical device.
- Customer service - responsible for the technical service and support of your product.
- Legal representative - responsible for any incidents regarding the medical device inside and outside of China and handling any recalls and other regulatory matters.
Some distributors are authorized to act as local agents. However, it is highly advisable to separate the legal and business sides. If the cooperation between you and your distributor is not satisfactory, this can lead to difficulties and high costs, as your partner will have to confirm the transfer of all responsibilities by signing official documents. Due to the size and fragmentation of the Chinese market, only a few distributors offer nationwide distribution. In all likelihood, you will need to find several organizations or start working with a partner with a larger distribution network.
You can also set up your own company in China, e.g. in the legal form of a Wholly Owned Foreign Entity (WOFE), and take care of setting up and managing the China business yourself. But that is a completely different story.
Conclusion: Entering the Chinese market is difficult and laborious, but once your medical device has successfully passed all the necessary tests and all the necessary approvals have been granted, the path is indeed clear. If your market study and strategy are correct, your company can expect strong growth and good business development in this demanding and promising market.
Frequently asked questions




