MEDICAL DEVICE DESIGN
Medical Design - Product Design in Medical Technology
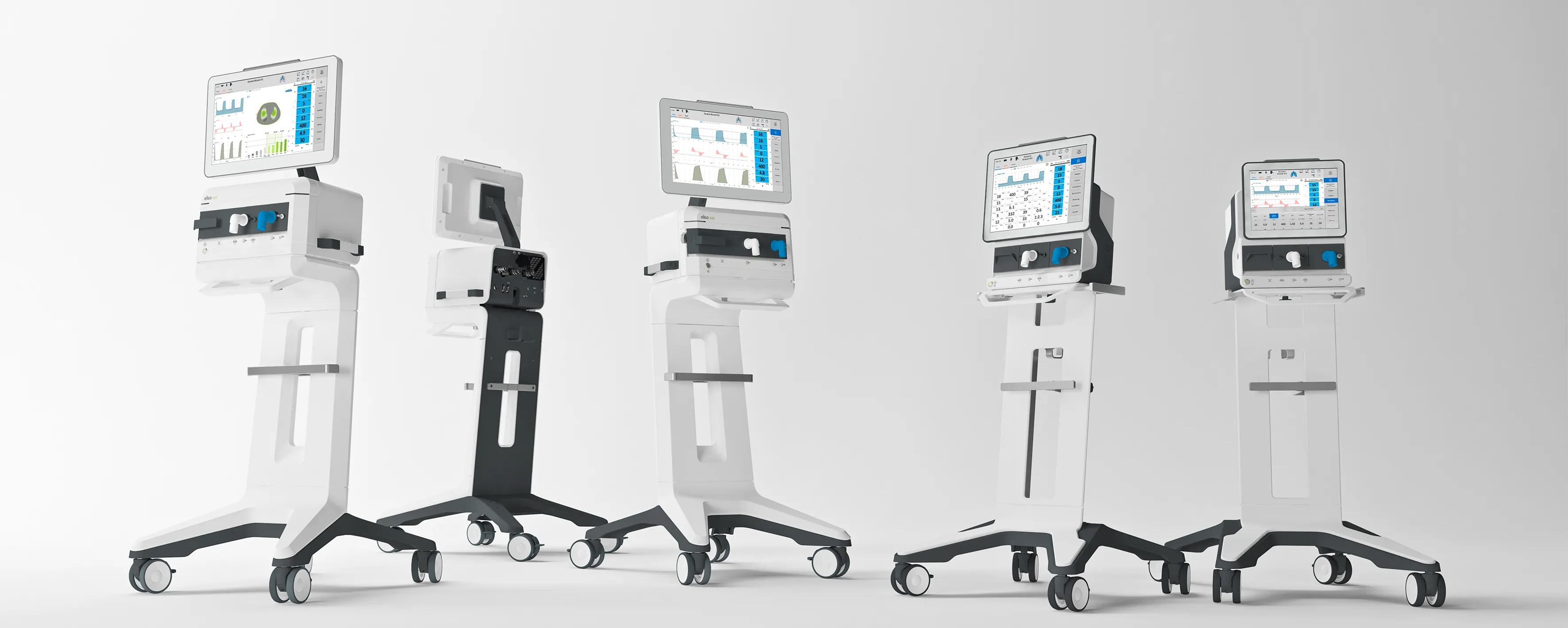

The key enhancement for your medical device!
You need more than a safe, CE-approved medical device to succeed in the global medical markets. Medical design makes the difference! Add value to your products to make them attractive to buyers, users and patients. Our experienced designers know how...
- Deep understanding of the basic requirements in medical technology & life sciences
- Knowledge of current regulations (MDR, FDA, NMPA), guidelines and standards
- Decades of experience in all relevant manufacturing processes in large and small series
- Research and application know-how to explore specific user requirements
Medical Design Services at a glance
Product Design: from the idea to mass production
From the idea to the concept, detailed design and construction to the transfer to series production - the product or industrial designer is responsible for the synthesis of function, form and user experience.
Product development in medical technology is subject to its very own laws. The product design process is subject to countless requirements, including risk management and numerous validations. We take into account safety and hygiene aspects, cost specifications and production technologies. This is only possible with experienced designers.
Omit the unnecessary and emphasize the important. This design principle results in simplicity, increased perceived value and a high level of acceptance among the target group. No-frills design reduced to the essentials, developed in a team with your in-house developers.

Medical Design challenges
that we solve every day.
Complicated workflows
clearly structured
Strengthen brand perception in the long term

Take into account the manufacturing process

Consider production quantities and unit numbers

Design cost-sensitive

Define color, material and surface

Comply with regulations

Ensure hygiene

Understanding multi-layered user groups

Meet ergonomics and usability standards

Safety through user tests

Develop user-centered Product Design
Rapid Prototyping: fast implementation of your visions
A full-size model says more than 100 pictures - even in medical technology
In the spirit of "a picture is worth a thousand words", we try to produce a sample for testing wherever possible and affordable. In the pre-development phase, these tend to be simple, inexpensive mock-ups. The further the product development progresses, the more realistic we can build the prototypes, right up to pre-series made from original materials with full functionality.
Rapid Prototyping can be quickly and flexibly fitted into the development process. Depending on the purpose, functional models, design models, partial or full prototypes are designed and procured based on our 3D CAD data. In close alignment with the test requirements for application tests or technical tests. You benefit from these possibilities, because problems and possible design errors become visible at an early stage and can be corrected cost-effectively.

Design for Manufacturing
Product development with an eye on production size, manufacturing processes and costs
For us, design is only finished when it has proven itself in mass production. As a technically oriented design office, we work with the aim of ensuring that our developments can be easily transferred to production. We lay the foundations for this at an early stage, as we check the technical feasibility and target costs as early as the proof-of-concept phase. Design for Manufacturing (DfM) is therefore integrated into the development process at an early stage.
A 20-year life cycle is no exception for medical products. This longevity is built into our innovation process. The surfaces must not age mechanically or due to disinfectants, the style does not follow short-lived trends and the product structure is easy to install and maintain.

Ergonomics & User Centered Design
We are often the "voice of the user" for our MedTech customers.
What do users really want? How can you find out? If you ask directly, you rarely get the right answer. In user-centered design, it is particularly important to know the application context for which the product is being developed. This includes not only the specific user target group but also knowledge of the spatial environment, the application and environmental conditions and the workflow. The mixture of observation, questioning and testing results in what we call design and user research.
In addition to regulatory usability, which is primarily aimed at safety and freedom from errors, it is the integrated ergonomics and user-centered design that make the products comfortable and convenient. They are essentially responsible for ensuring that users find the products pleasant and enjoy using them - thanks to a positive user experience.

3D Visualization & Animation
Good images create common objectives and help to convince.
Visualizations and animations can be easily generated from our 3D CAD data at any time during the development process. This is also referred to as project marketing, because the progress and benefits of projects can be easily communicated using illustrations. And at almost no cost, because the 3D CAD data already exists.
Animated videos that can demonstrate and visually prove the functionality and effectiveness of the products are used for pre-marketing. Right up to hyper-realistic renderings that could ultimately also be used in commercials.

Packaging, Labeling & IFU Design
Complete solutions for your product.
Packaging and labeling are essential components of the product experience for sterile or safety-critical products and are part of our responsibilities as medical designers in MedTech.
The design of instructions for use, brief instructions and quick-start guides, together with suitable training measures such as video tutorials, are an important prerequisite for error-free operation by medical staff and patients. And therefore just as important for CE/FDA approval.
We will be happy to advise you and work with you to find the best combination of measures that lead to the desired effect and are compliant with regulatory requirements.

Strong partnerships with industry experts - mastering every challenge in medical device development together! We rely on cooperation - because it is the key to success!
Frequently asked questions
When is the right time for design?
The design should be integrated into the development process as early as possible. Early integration enables user requirements, ergonomics and functionality to be optimally taken into account. This ensures that your product meets the needs of the user, offers ease of use and minimizes errors. You can also achieve an optimum balance between function, form and costs right from the start, which saves time and resources.
How do I ensure that the design meets all regulatory requirements?
By integrating current regulations such as MDR, FDA and NMPA into the design development process from the outset and taking safety and hygiene aspects, risk management and validation into account. Working with experts helps to ensure compliance and facilitate the approval process.
How do I find the right balance between function, form and cost in design development?
Through holistic planning in which material selection, manufacturing technologies and production quantities are taken into account at an early stage. Iterative design processes and close collaboration between designers, engineers and product managers help to develop a functional and appealing product that is also cost-effective.
How important is user-centered design in medical technology?
User-centered design is crucial for developing products that meet the actual needs of users. It increases acceptance, improves user-friendliness and reduces the error rate. By understanding the application context and user requirements, you create products that are comfortable and efficient to use, which ultimately contributes to the success of your medical device.
How can Rapid Prototyping improve the design development process?
By creating prototypes quickly, you can visualize and test design ideas at an early stage. This enables potential problems or design errors to be identified and rectified at an early stage, saving time and money. Rapid Prototyping promotes iterative development and improves product quality through direct feedback from users and stakeholders.

"From briefing to market launch - we are always at your side. As partners you can rely on - even when things get complicated!"