Usability Engineering
Usability Engineering in Medical Technology
User-friendliness and regulation
Usability engineering in medical technology combines regulatory requirements with optimal user-friendliness. The legal requirements ensure the safety and effectiveness of medical devices, while user-centred design enables their intuitive and efficient use. This combination is crucial in order to develop successful products for the benefit of users and patients.
The usability of a product is only as good as its integration into the development process!
In order for usability engineering methods such as context analysis or formative evaluations to lead to an actual improvement of the product, they must be embedded into the project at the right time and with the appropriate questions. By synchronizing usability engineering and design development, we have made it our goal to effectively incorporate the needs of the user into the overall development and to achieve optimum user-friendliness with a high level of patient safety.
Why you should integrate usability into your process :

Differentiation from competitors

Safeguarding the summative evaluation

Safeguarding decisions

Regulatory compliance

Risk minimization of the medical device

Basis for the accompanying documentation

User-Centered Design
In medical technology, interaction design and product development create solutions within the problem space previously identified and defined by usability. By coordinating the processes of technical development, product design, digital design and usability engineering, the disciplines complement each other perfectly. By using the right interface methods from our medical design process, usability engineering becomes an effective tool for creating user-friendliness and not just a time-consuming mandatory regulatory event for your medical device documentation.
Usability Frontend & Backend
In addition to process integration, the accessibility of findings and results from usability activities is another decisive factor for the effectiveness of usability engineering. Project participants often have to fight their way through unwieldy usability documents, i.e. the "usability backend", in order to access the information relevant to them. These formal documents are of course relevant for the usability file required by the MDR and FDA (Human Factors). However, they are not always suitable for the day-to-day work of project teams or product managers.
A "usability front end" in which content and findings are made usable for the respective needs of the project participants is therefore the key to success. In this way, ergonomic findings can be turned into a common basis for communication and an interface between the various stakeholders in medical technology.
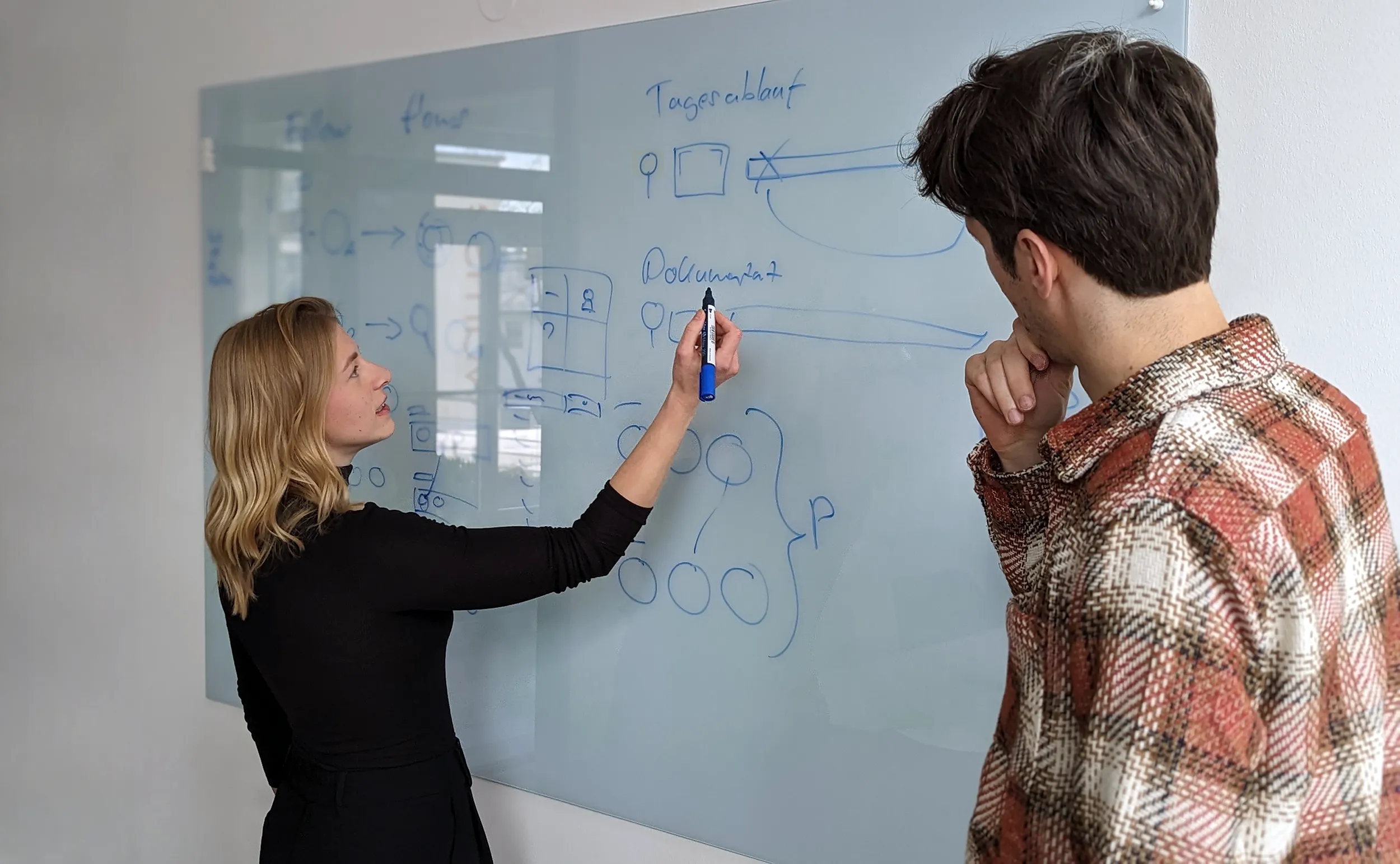
Usability Engineering Services
Usability integration into the design process
Usability from the very first idea! We offer continuous support for the design process through usability engineering, allowing ergonomic findings to be integrated into your product development as early and directly as possible.
To this end, our design services mesh seamlessly with usability activities to help capture and develop human-machine interaction in a structured way.
Usability thus becomes a lively part of the development process, facilitates decision-making through quick prototypes and secures conceptual and technical development.

Usability Strategy & Management
The usability strategy as a secure foundation for your project. Together with you, we plan and support your tailor-made process as the cornerstone for effective and goal-oriented usability activities that support your development. At the same time, we synchronize usability with design development and ensure that the right test prototypes are available at the right time during development.

User Research & Context Analysis
Know your users and understand their problems! In medical technology, this is a matter of course and part of the everyday work of designers. By involving users and methodically capturing the context of use, we support you in gaining objective insights and defining the usage requirements for your product. Wherever possible, our designers go on site to at least take a look at the application in the operating theater or intensive care unit. This usually results in systematic research and context analysis.
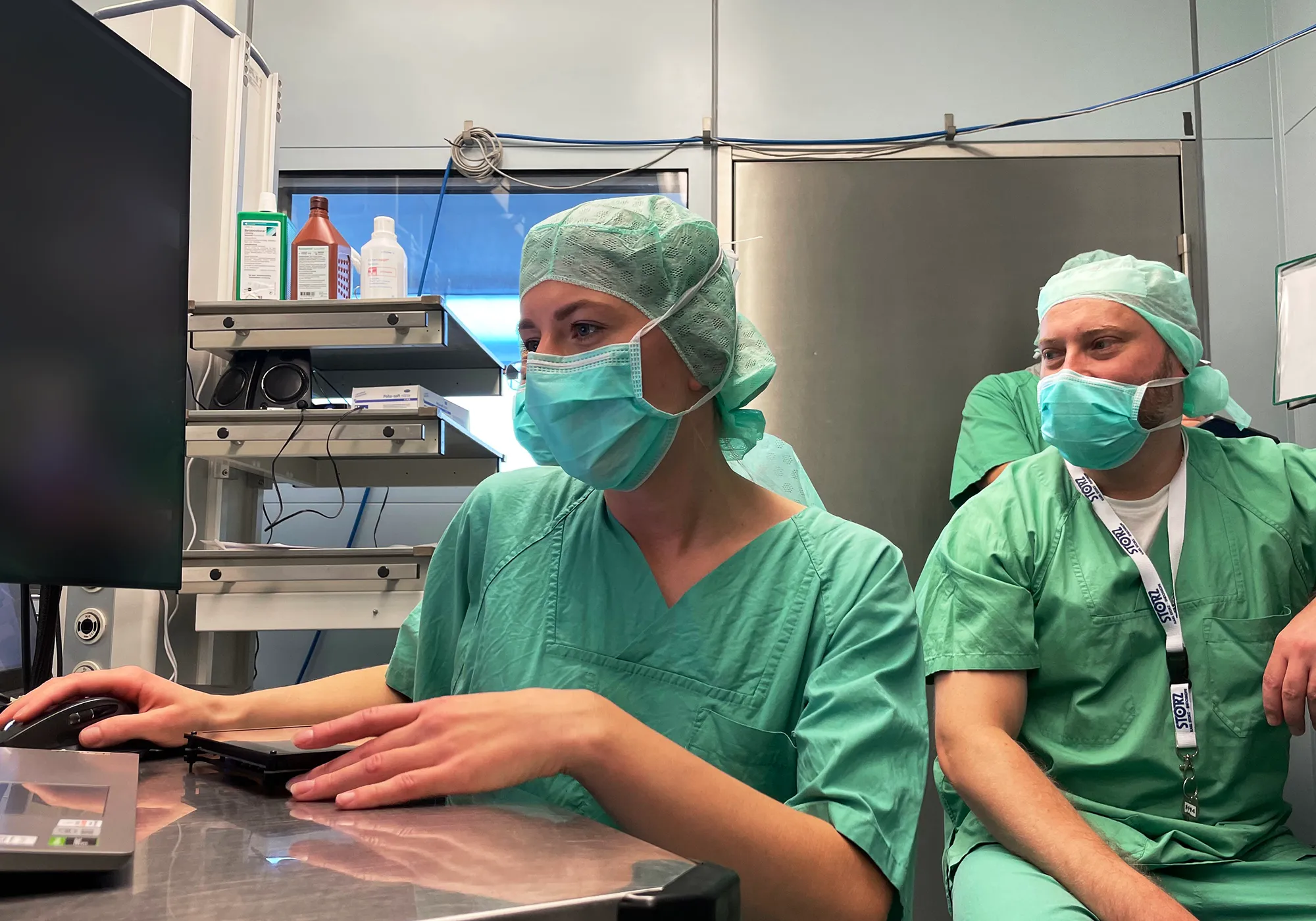
Usability Engineering File & Documentation
The Usability Engineering File (UEF) is a central element in the development of medical devices in accordance with the IEC 62366-1 standard. It documents all activities of the usability engineering process and ensures that usability is systematically planned, implemented and checked in formative and summative evaluations.
With a comprehensive usability engineering file, you not only meet the regulatory requirements, but also improve the safety and effectiveness of your medical device. We support you from the identification of user needs, through use scenarios and the use specification, to user-related risks for risk analysis and validation of usability, and ensure that all necessary regulatory documents are created in full and in compliance with standards.

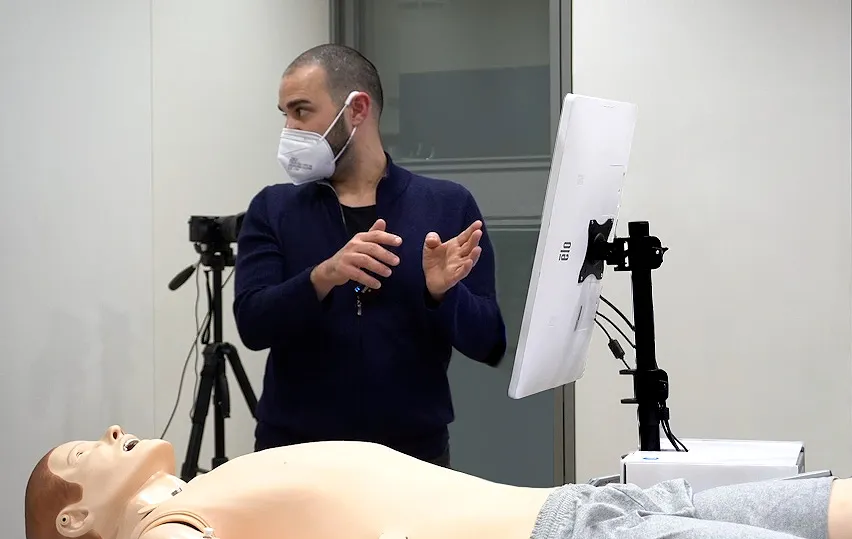
Formative & Summative Evaluation
The proof of the pudding is in the eating - tests are the best way to ensure the quality and functionality of a product. Simulating or reproducing the user experience in usability tests provides valuable input for design development. This is why we work with you to carry out formative and summative evaluations during the process in accordance with the MDR and FDA. This validates usability, particularly with regard to safe use.
Frequently asked questions
Why is usability engineering so important for the design development of medical devices?
Usability engineering ensures that medical devices are intuitive and safe to use. Poor usability can lead to operating errors that jeopardize patient safety. By incorporating usability principles at an early stage, user needs are recognized and integrated into the design, which increases the effectiveness and safety of the product.
How can the integration of usability engineering into the development process ensure product success?
Usability combined with a suitable design are often the only real differentiators of successful products on the market. By integrating usability engineering from the outset, you ensure that your product is not only functional, but also user-friendly and appealing. This increases user acceptance, strengthens customer loyalty and gives you a competitive advantage, which ultimately ensures the success of your product.
Can I also use usability engineering productively outside of regulated medical devices?
Yes, usability engineering is universally applicable and can be used in any product development. Regardless of the industry, it helps to improve user-friendliness, reduce errors and create a positive user experience, which promotes the overall success of the product.
How can the synchronization of processes ensure that the appropriate test samples are available at the right time?
Development processes can be synchronized through close coordination between designers, engineers and usability engineers. This ensures that prototypes and test samples are available in good time to carry out the necessary tests and evaluations. This synchronization makes it possible to obtain user feedback at the optimum time and incorporate it directly into development, thereby increasing efficiency and product quality.
How can I ensure that usability engineering contributes to accelerating the project?
By integrating usability engineering at an early stage, potential problems can be identified and rectified at an early stage. Formative evaluations during development enable iterative improvements, which avoids costly changes later on and makes the development process more efficient overall.

"A product that inspires in its use - effective process integration makes usability a powerful tool in your product development."
Your contact person for usability engineering
Anna-Lena Gölz
Head of Usability Engineering
+49 89 780 196 50
annalena.goelz@wilddesign.de
