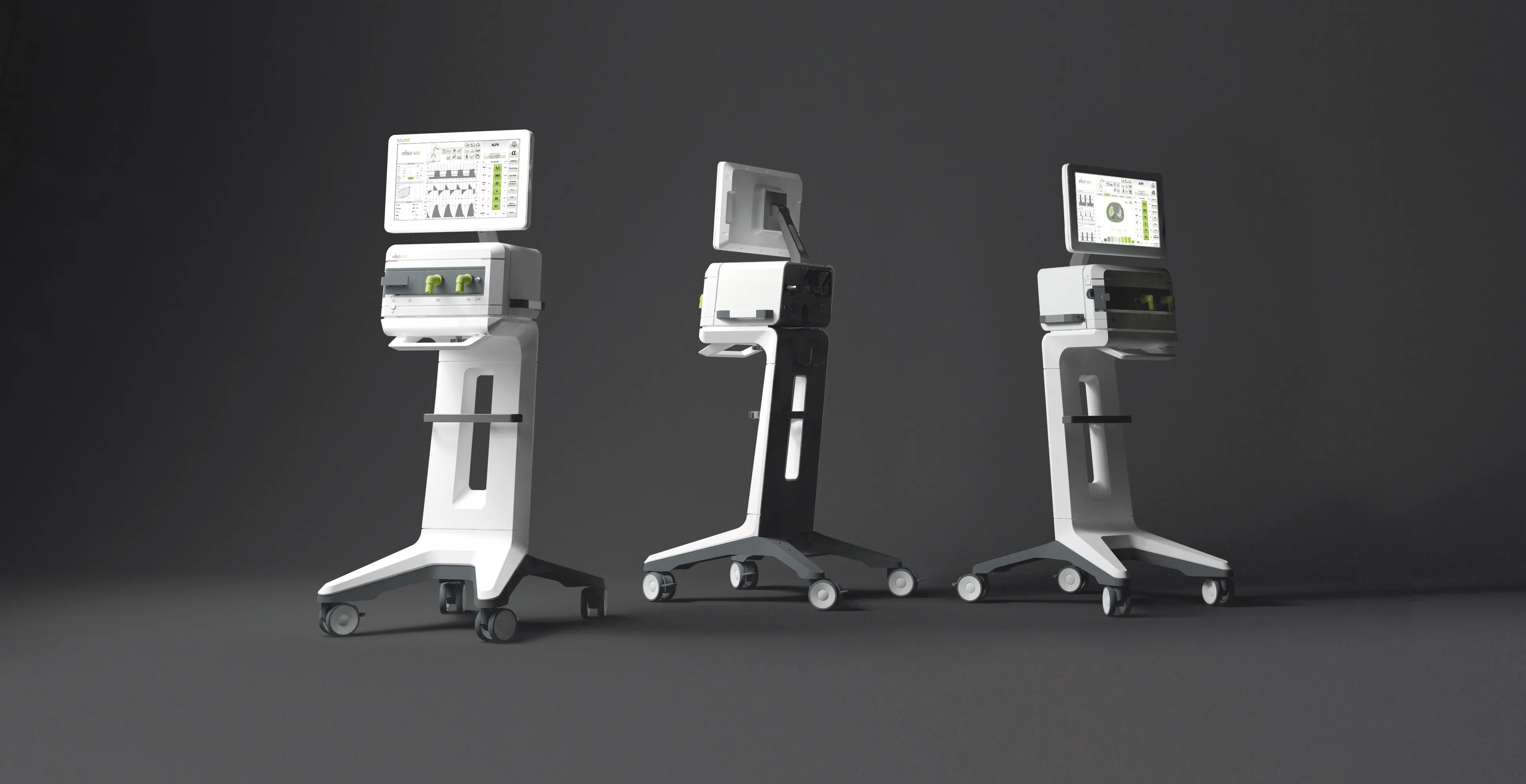
Expertise
Developing medical devices correctly - with usability and design
The original article was published in November 2015 in the trade journal MTD (Medizin-Technischer Dialog)

Authors:
Marc Ruta, Dipl. lndustrial Designer, Wilddesign GmbH & Co. KG (Gelsenkirchen, Munich),
Michael Engler, Dipl.-lnformatiker (FH), Michael Engler IT-Consulting (Essen)
Time pressure, different languages, a variety of operating concepts, a growing amount of available information - while for decades powerful sales machinery was able to guarantee success in the global medical technology markets, strategies are changing under the increasingly perceived threat of incorrect operation and the cost pressure felt everywhere, and two new recipes for success are crystallizing:
1. usability - leads to safe products that are suitable for use.
2. design - leads to simple, smart and attractive products.
Combined with solid and cost-oriented development, these two factors can create superior product concepts, especially in medical technology, that are welcomed by the markets with open arms.
Why usability?
Usability plays a central role in today's medical device development process and, as defined by ISO 9241-ll, describes the degree of fulfillment with which users achieve their goals effectively, efficiently and satisfactorily. Other important aspects are added by the user experience - it should also be a certain pleasure to use the product not only for its intended purpose, but also as a positive experience before and after use.
The fact that a good usability design also brings tangible benefits such as faster purchasing decisions is actually obvious, but often not realized. Manufacturers can also credibly demonstrate that user productivity increases or that training costs are reduced. These are things that customers definitely appreciate.
Recognize user requirements and goals
A separate profession has now been created for this: the usability engineer. Even if the developers in their laboratories make every effort to anticipate user behavior patterns based on their experience, they will hardly be able to reliably predict the requirements of the users of their product without ever having spoken to the users themselves or having watched them carry out the intended operating procedure. A methodical approach must be used so that user-related needs and requirements can be determined effectively and a manufacturer can achieve competitive advantages.
The usability engineering process
A usability engineering process is prescribed by standards for medical devices, as operating errors can lead to injury or, in extreme cases, even death of the patient. The harmonized standard IEC 62366:2007, which is closely linked to the risk management standard ISO 14971, prescribes a usability engineering process that focuses purely on safety. The usability engineering process is often not very popular with manufacturers, as the necessary knowledge is not available within the company.
Manufacturers can also refer to ISO 9241-210 to compensate for the benefits and gaps in the standard that focuses purely on safety. Like all steps in a product development process, the activities of the usability engineering process must be planned so that sufficient time and resources are available.
First, the usability engineer analyzes the context of use, which includes the users, their tasks, work equipment and the relevant environment. Examples of this include making a diagnosis, performing an operation, training users, carrying out therapy in a private or clinical environment, considering other medical devices or tools, etc.
User experience is important
This knowledge is often only available to patients or medical staff who experience and use the product directly. It is essential to efficiently interview the right people or to observe them performing the task. Only when these findings are available can usability engineers derive the needs of the users and define the usage requirements. This results in reliable requirements that can be implemented during development and design.
Role of product design
No user wants a complicated product! Simplicity and order are two of the basic principles of good design. Although these are not regulated in any standard, the so-called soft product factors also make a decisive contribution to product acceptance. Medical design and usability design are sub-disciplines of contemporary medical device development and must be effectively integrated into the development process.
The product manager must be able to define the basis on which the medical designer develops his proposals. Requirements management is the key word. An effective method from our practice is the "24 design factors", with which not only the technical, normative and user-required aspects are explored, but a holistic view of the future of the new product in the context of the brand can be taken.
Designer as mediator
The designer acts as an interdisciplinary mediator here: Research & Development provides the technological specifications that give the product its justification as an innovation. Marketing will name benchmarks that are representative of the target segment, and the clinician will formulate requirements from the user's point of view with the help of the usability engineer. What other factors are responsible for the design of a medical device?
Other ways of thinking and seeing things
By opening up the view of existing patterns of thought and action, the designer creates new perspectives on already solved or unresolved issues. They should therefore be involved in the project as early as possible, as they can summarize market trends, the brand's communication intentions and the needs of users into a comprehensible vision and visualize the product idea, which can be used as a guide for all those involved. For example, the "intelligence" of the products plays a primary role today because users deduce the operation and intelligence of the medical devices from their familiar handling of their private smart devices.
However, marketing and market specifications also influence the appearance of a product. In addition to possible specifications from the corporate design of a brand, effective patents can stand in the way of the desired device concept. Technologies are also subject to user acceptance and can cause attractive designs to fail if they do not meet expectations in terms of size, material properties, volume, weight, vibration, flexibility, etc.
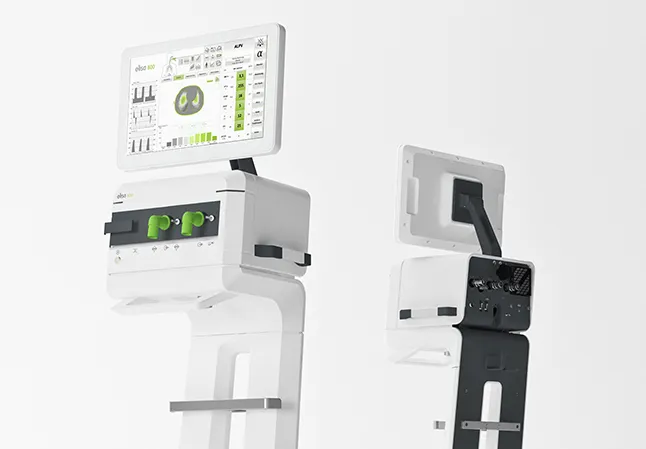
Studies during the development phase
The acceptance of many features of the product can be tested before the end of development. This is where usability studies with users during product development - so-called formative usability studies - can help. They provide feedback for developers and designers before it is too late and a product urgently needs to be launched on the market. This requires a frequently repeated procedure, which considerably reduces the risk of faulty development.
Integration of design and usability
If the development team and investors need to be convinced of the product idea, the medical designer can make the technical description of the project "tangible" with a visualization and align everyone involved with the goal. There can then be a seamless transition to the concept studies and further development steps. The usability engineer should be brought into the project even before the concept phase begins.
As soon as the medical purpose requires an intended application through human actions or the use of tools or devices, the usability engineer must examine the conditions of these applications and formulate requirements for the design of these processes and devices that ensure safe and satisfactory use.
Include software too
This increasingly applies to software development. IEC 62304 stipulates that usability requirements must be incorporated into software development - but the requirements for maintenance, storage, installation, transport and disposal must also be determined in accordance with standards.
All disciplines involved must effectively formulate and demand their requirements for development in order to create a successful medical device. Usability engineers and medical designers become central designers in this area of conflict.
Article published in the trade journal MTD (Medizin-Technischer Dialog),
November 2015 issue
-> to the Salvia Medical case study
Frequently asked questions