Experience
Medical technology: China - from imitator to innovator
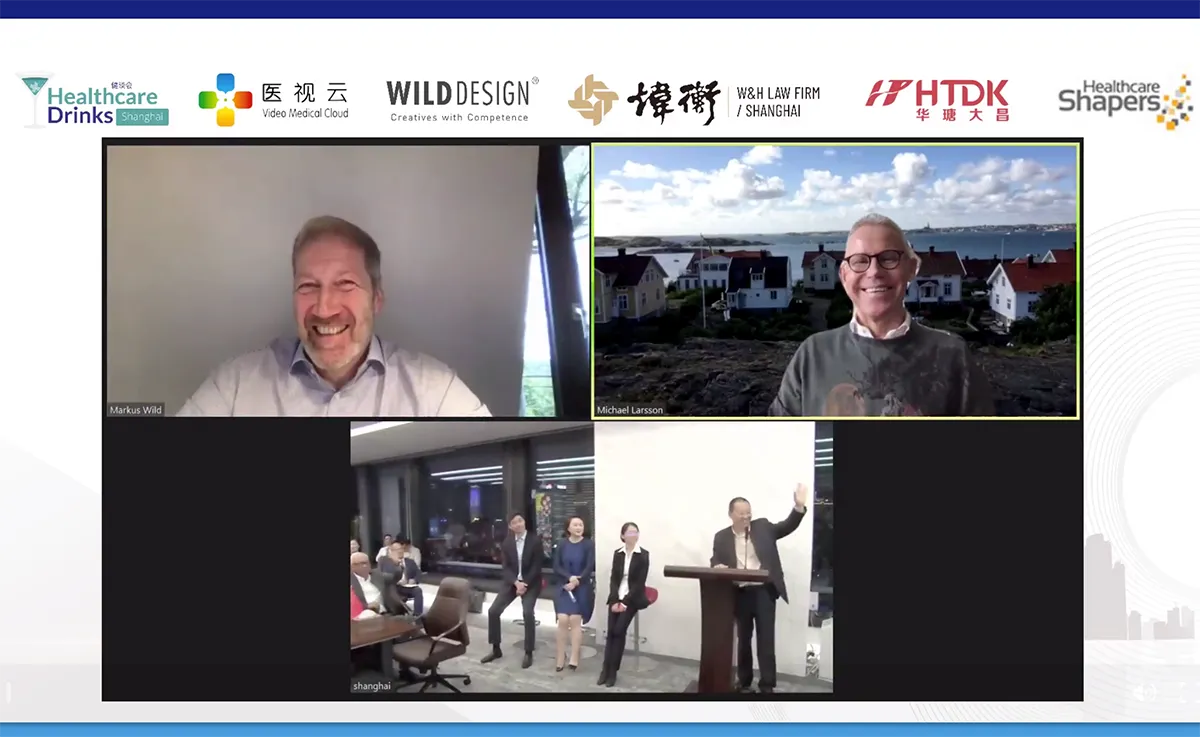
For the virtual MEDICA/COMPAMED in November, we organized an exciting panel talk with Healtcare Drinks - a networking group that organizes events around the world and focuses on the Chinese healthcare industry. In addition to our CEO Markus Wild, we were joined by: CEO of Medela AG Michael Larsson, COO Glendy Wang from MicroPort, Managing Director Min Fang from Warburg Pincus, Head of Regulatory at PerkinElmer Nancy Meng and moderator of the panel talk and Managing Director of HTDK, William Jin.
The result: an exciting exchange and discussion of topics and trends in the healthcare industry from a European and Chinese perspective. Reason enough to reflect on these here.

China recovers faster
2020 was a year of many changes and challenges - especially in the healthcare sector. While innovative products, vaccines, masks, ventilators and laboratory equipment were more in demand than ever before, other areas of medicine were pushed into the background. These included orthopaedics, cardiology procedures and elective operations in general - including the resources and supply chain requirements behind them.
While celebrations, face-to-face meetings and a largely normal everyday life are now possible again in China, the numbers in Europe continue to rise and there is still a lot of focus on online communication and commerce. In this context, the predictions of our CEO Markus Wild from the first days of the COVID-19 pandemic are also interesting to read.
The situation in the markets is similar: medical technology companies are increasingly focusing on projects in China because the markets there are recovering faster. In the area of new approvals for medical devices, medical ventilation products are leading the way. The large volumes make it much easier to amortize innovation costs. On the other hand, the strength of Chinese companies lies in the online sector anyway. The strategy for the next few years? Investments in China and South East Asia. Various medical sectors have also grown strongly in Europe in the past year, especially digital health.
Localization: China and Europe
International logistics processes are complicated to manage, especially in times of coronavirus, the risk of failure is high and prices have risen dramatically. The pandemic has further fueled existing trends towards local production, which also has a positive impact on the environment. A key factor on site is the staff, as they understand the local culture. For good cooperation, partner companies should have an understanding of both Western and Eastern cultures, such as our team in Shanghai.

Local knowledge is crucial, especially for joint ventures - and Chinese companies are therefore already expressing great interest in M&As with Europeans. A keyword for success in China is convergence. The experts on the panel agree that Chinese products in the premium sector are approaching European characteristics in terms of quality, as processes and quality are quickly optimized. Follow the master - but what comes next once you have caught up with the master?
Balancing standards and the economy
Interest in and demand for Chinese markets have increased on all sides - even or especially in times of crisis. The new investment agreement between China and Europe is intended to facilitate access to Chinese markets in the future. How is market access in China being accelerated? Regulations and corresponding control mechanisms are much stricter in the EU and are not easy for Chinese manufacturers to understand. This is particularly true of the new MDR approvals: they take a long time and are costly - the rules for medical devices are a challenge, especially for small and medium-sized companies.

Due to the time-consuming preparation of existing products for MDR, innovative projects in Europe have been put on hold for years - an innovation backlog. Business in China is therefore perceived as a temporary way out: The path from idea to market is much shorter. The RCEP trade agreement between China and some Asia-Pacific countries also facilitates business activities. This should further strengthen the status of the Asian region: Growth opportunities are being sought after the pandemic. Standards for working conditions and the environment have not been set.
On the other side: EU standards that on the one hand make medical technology innovations more difficult, but on the other hand are supposed to ensure fair conditions. Does it have to be either/or? An expansion of international cooperation for fairer conditions everywhere - with our locations in Germany and Shanghai, we are contributing to a solution.
Frequently asked questions