Expertise
Innovation vs. optimization in medicine - an innovation workflow
Innovation in the eyes of the world
When we talk about innovation, we first think of scientific research that enables a new technological function or changes an existing behavioral pattern. This change is accompanied by a correspondingly high financial return.
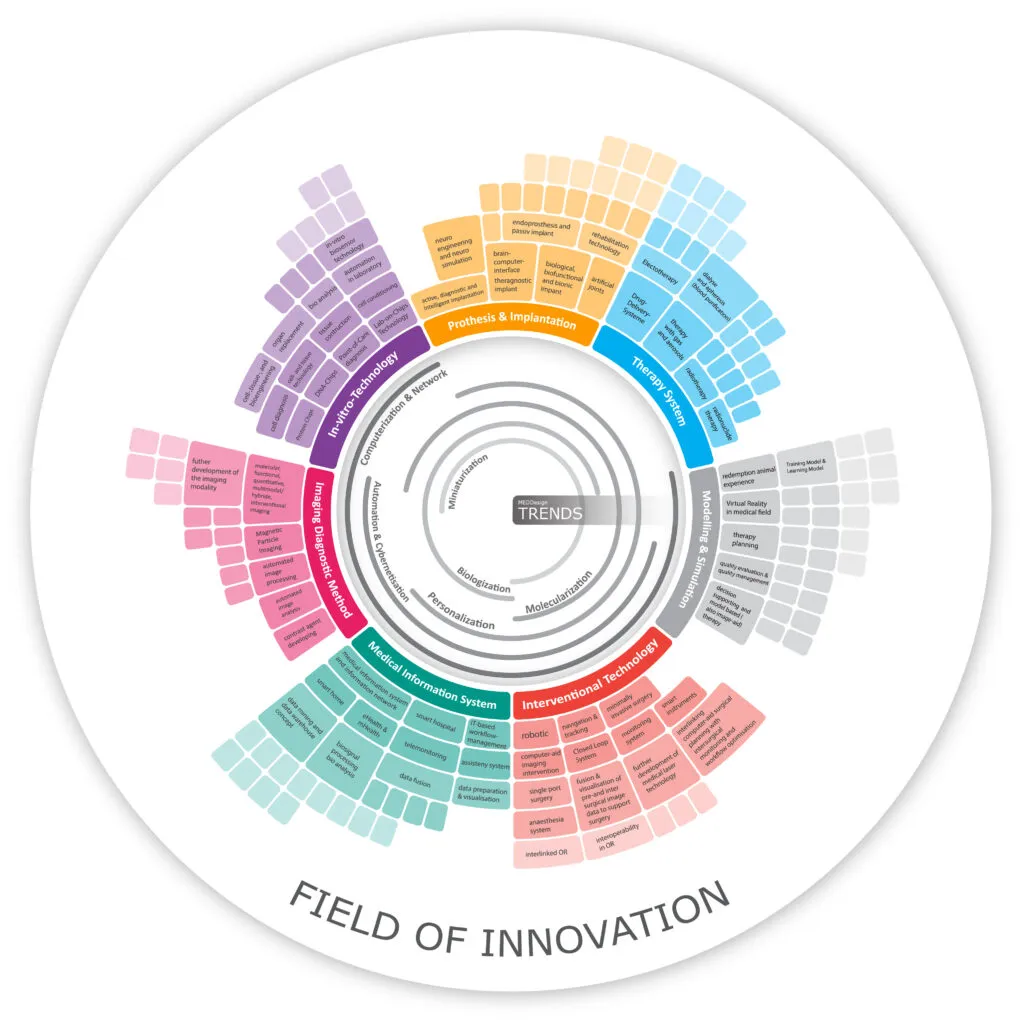
Real innovation is disruptive
True innovation means breaking out of the existing system, thinking outside the box and solving problems. Furthermore, true innovation involves transforming solutions into usable tools or products that help users avoid mistakes and provide an optimal user experience.
Innovation is when you are the first to understand exactly what features of the product have the potential to create additional or alternative market value and then build it. An innovative idea does not necessarily mean that the costs for product development are high, but it must be revolutionary when it is launched.
Innovation and optimization in the healthcare sector
An R&D professional constantly moves back and forth between creating innovations and carrying out optimization work. He/she finally creates a good product after going through several optimization processes.
The innovation of medical devices is often based on new medical treatment protocols and results in devices that comply with these treatment methods. New medical devices offer better treatment and more comfort: they increase success rates, reduce risks and avoid errors in the treatment of injuries and diseases, control costs and extend the life of the product. These are all well-known benchmarks for measuring product innovation and optimization progress in the medical field.
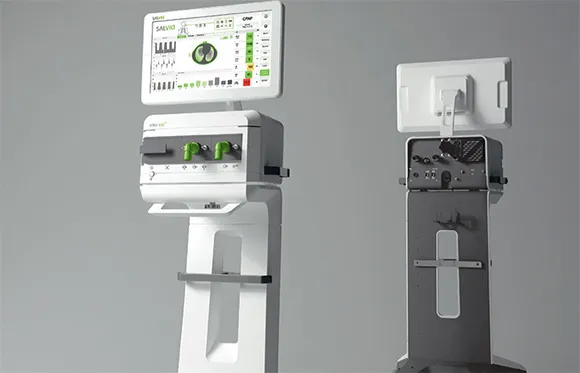
The innovation itself means overcoming an existing product and bringing more advantages and benefits, e.g. the ELISA ventilator from Löwenstein, Germany, which offers an optimized process for comprehensive emergency ventilation and gives doctors more time to treat critically ill patients.
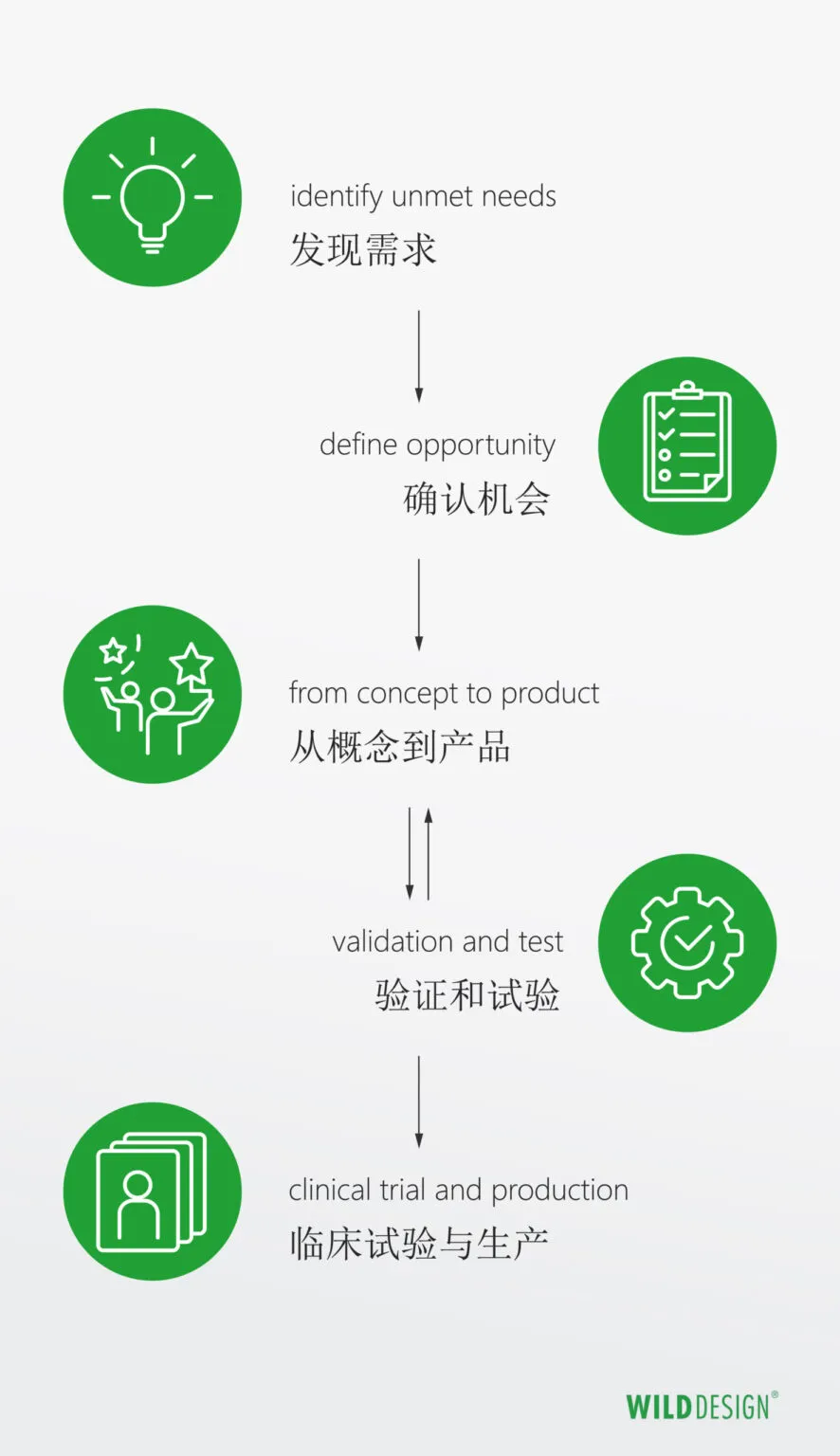
How can we do research and development properly?
We don't need to reinvent the wheel, but we do need to revolutionize its purpose. So how can we find revolutionary ideas?
An established manufacturer of medical devices invests a lot of money and personnel in research and development. If you divide product development into different phases: "from initial ideas to concepts, to mature solutions, to final product design and finally to validation and testing". Then these phases are predictable, achievable and verifiable through planned execution.
Every company has clear process guidelines and evaluation criteria for its development work. The development of medical devices, especially Class III medical devices, is subject to strict safety and efficacy requirements that require high investments and a corresponding amount of time for testing.
The purpose of manufacturing tools is to reduce risk, simplify workflows and reduce human error. Once a medical device is in the development process, there is no room for "error" in clinical use.
A manufacturer of surgical tools must provide the surgeon with highly reliable devices that can improve the outcome of an operation. Product reliability is quantitatively comparable, e.g. for coronary arteriosclerosis, 90% is the highest success rate currently achievable; for general surgery for gastrointestinal cancer, the success rate is between 95-99%. There is a data standard with which we can scientifically quantify the reliability of a medical product.
For the doctor, a reliable device is an excellent aid, but for the patient, any failure can lead to a worsening of the situation or death. It doesn't matter if the device is 95% or 99%, reliability does not reduce the patient's concern for their condition. When we develop a treatment concept, it is important to think about the patient's feelings.
When does innovation actually take place? If we make our products "perfect", with a reliability that is infinitely close to "100%", is that innovation? It means we are doing a good job of optimization, but it doesn't necessarily mean we are doing something innovative. During the R&D process, we have to constantly weigh up whether we want to invest resources in optimizing existing products or whether we want to change them completely.
WILDDESIGN specializes in helping healthcare companies design their innovative products of the future and offers services to optimize product development. If you would like to find out more about how we can help you develop better medical products and challenge the promising future of the medical market, please contact us and we will be happy to talk to you!
Frequently asked questions




