Perspective
Medical technology made in Germany: has the peak been reached?
German medical technology companies are considered innovation leaders worldwide. The Association of German Engineers (VDI), in its current brochure "Theses and fields of action" the industry remains on course for growth. The industry report on the healthcare sector predicts annual sales growth for companies in Germany in the future - it is expected to reach around 90 billion in 2020. According to the BMWI, the healthcare industry recorded higher growth than the economy as a whole for the fifth time in a row in 2015. So are the prospects for the sector bright?
Yes, but no reason to put your feet up. It is true that demographic change and greater health awareness are leading to a continuing increase in demand. However, being number 1 is not only a distinction, but also an incentive: How can we maintain our top position in the future? What could cloud the good prospects? In particular, the VDI identifies the risks in increasing regulation, growing complexity and increasing cost pressure. The association is therefore appealing for further tightening of the framework conditions for new approvals to be avoided. This is the only way to maintain short development times and the rapid market launch of innovative medical products.
Innovation - the engine of the industry
However, the VDI sees innovation as the driving force behind medical technology. It is also seen as an opportunity for further growth in the future. As designers, however, we are increasingly noticing that in addition to technical innovation, the needs of the user are also becoming a field of innovation - an area with a lot of catching up to do.
Technical vs. application-oriented innovation
As developments in the industry are progressing at such a rapid pace, I believe we need to differentiate between technical and user-oriented innovation in the future. Until now, the term has been used exclusively to refer to technical innovation and thus to advances in biology, physics, biotechnology, etc. The fields of innovation defined by the VDI - diagnostic imaging procedures, interventional techniques, in-vitro technologies, medical information systems and telemedicine, modeling and simulation, prostheses and implants as well as therapy systems - are also exclusively of technical origin. However, their importance will diminish in the future in favor of user needs.
Application innovations in the areas of user experience, usability and design on the other hand, will gain in importance and make up the true innovative value of a product. Innovations will therefore remain the engine of the industry, but the big trend is in the area of user-friendlinesswhile the technical lead can hardly be extended.
The VDI sees particular growth in the 6 trend areas:
- Miniaturization
- Computerization and networking
- Molecularization
- Biologization
- Personalization
- Automation
As developers in medical technology, these trends also concern us in our daily project work. However, we are also noticing other trends. For example, the new understanding of health and the associated merging of classic medical technology and consumer products is particularly noticeable at the moment. People want to gain more and more control over their health and fitness and need appropriate medical devices to do so. Body analysis products, wearables and the like are moving into the home environment and at the same time placing extremely high demands on us designers.
The products must be able to integrate visually into the home environment, but operation must be absolutely simple and safe. Another important design criterion: the user must be familiar with the application; they want to be able to use the medical products in the same way as they are used to with their other technical devices, especially their smartphone.
The user assumes that the product must satisfy all safety aspects and carry no risks whatsoever. But to be truly successful, the product must be accepted by the user. And this is exactly where we designers come in: by researching trends, optimizing usability and advising on the overall concept.
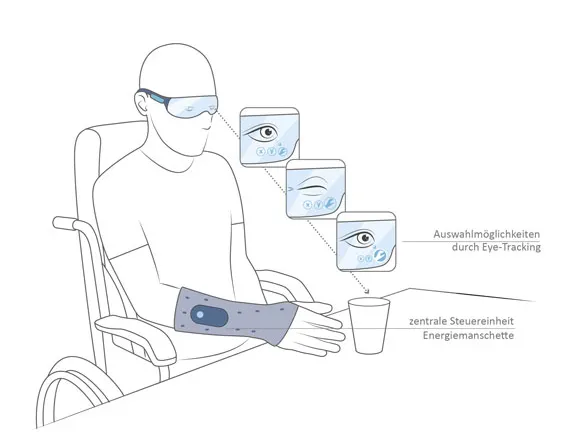
The VDI cites the interaction of technical devices with the human body (MMI human-machine interfaces) as a particular challenge - the primary aim here is to avoid operating errors and other undesirable reactions. This is particularly complicated in the case of implants. What the designer can achieve here is presented in our Intakt project project.
Together for success: interdisciplinary teams for a tailor-made product
From the initial idea to mass production, many other experts are involved in the development of medical devices alongside us designers. Engineers, economists, computer scientists or marketing specialists - everyone brings their own perspective and individual requirements to the product. The art is to filter out the essence from these different teams. In addition to designing the product, we also take on the role of moderator and mediate between the respective specialists.
What qualifies us for this? We have always been used to bringing design into an interdisciplinary context and optimizing it with regard to various requirements. This interdisciplinary work, in which many specialists contribute their knowledge at the edge of their specialist field in order to develop the best possible product, is what makes this industry so extraordinarily exciting for engineers and designers.
I fully share the view of the VDI. After all, medical technology also offers us designers an interesting, challenging and creative spectrum. With the start of regulation of usability around ten years ago - one of the most influential trends trends in medical technology - our part in the product development process has once again taken on a special role. This is because it focuses on the user and their specific needs.
And it is precisely these requirements that can be met by designers. Because we don't just want to make a product more beautiful, we want to make it better. We see the standard requirements, for example according to ISO 9241 or 92366, as an opportunity to develop a medical device in such a way that it is immediately understood by the user. Only then is it a good product. And this is precisely the key to continued success for the medical technology sector.
How will these and other trends in medical technology keep us busy? This article will give you some interesting insights: Technology trends in medical technology
You can already look forward to more news on this, as the update of the Medical Trend Report will be published shortly - with new trends, interesting developments and many interviews with experts from the industry.
Frequently asked questions